
TABLE 1
JAMES F. BELL III
Cornell University, Department of Astronomy, Center for Radiophysics and Space Research, Ithaca, NY 14853-6801 USA
Citation: Bell III, J.F., Iron, sulfate, carbonate, and hydrated minerals on Mars, in "Mineral Spectroscopy: A Tribute to Roger G. Burns," Geochemical Society Special Publication 5 (M.D. Dyar, C. McCammon, and M.W. Schaefer, eds.), pp. 359-380, 1996.
Abstract--The Martian surface is a mixture of highly oxidized secondary minerals and relatively pristine basalts. The currently-known and inferred mineralogic inventory provides evidence for possible large-scale climatic variability during past epochs. Because they are often formed under unique environmental conditions, the most diagnostic minerals that can be used to assess the duration and magnitude of past climatic changes are ferric oxides/oxyhydroxides, sulfates, carbonates, and hydrates. This paper (a) reviews the current telescopic and spacecraft observational data and interpretations regarding the occurrence of these phases on Mars; (b) presents some new results and interpretations from visible to near-IR telescopic observations of Mars obtained in 1990; (c) outlines some new models for the weathering and alteration of surface materials that are consistent with the available data; and (d) defines a set of observational measurements (telescopic, spacecraft, and in situ) that can be used to discriminate between possible weathering and alteration scenarios, as well as to provide important new constraints on the composition and mineralogy of the Martian surface.
To those who use spectroscopy to study the mineralogy of planets, satellites, and asteroids, Roger Burns was the person who literally wrote the book that defines our field (BURNS, 1970, 1993). This extraordinary book, which was revised and updated by Roger just prior to his death, is required reading for every graduate student entering the field, and is an essential element of the reference libraries of the many professionals who have gone on to pursue careers in planetary spectroscopy over the past several decades. In particular, Roger's detailed theoretical treatment of absorption features in visible to near-infrared spectra of Fe-bearing phases like pyroxene, olivine, and iron oxides spurred a generation of observational astronomers to (successfully) search for the occurrence of these minerals on other solar system bodies. Beyond this initial impetus, Roger also provided consistent and valuable theoretical and laboratory guidance throughout his prolific career on the interpretation of the spectral features that were detected.
Mars was a particular favorite of Roger's, and was the focus of much of his non-terrestrial research. Specifically, Roger realized the potential importance that a small number of spectrally-active minerals could have towards elucidating both the current and the past chemical weathering environment of the red planet (e.g., BURNS, 1980, 1987, 1988, 1993). To Roger, the question "Why is Mars red?" demanded an answer not only dealing with which specific minerals are responsible for the color of the surface, but also with which specific surface weathering or alteration processes can be inferred based on the current mineral inventory (e.g., BURNS, 1993; BURNS and FISHER, 1990, 1993).
Roger was an enthusiastic supporter of efforts to obtain new spectroscopic observations of Mars at key wavelengths, and his excitement with new or controversial discoveries was especially inspiring to young researchers. His probing comments, gentle criticisms, and insightful suggestions for follow-up investigations are keenly missed among the planetary science community. While he would certainly have blushed over such praise and the attention that his research is receiving in this volume and elsewhere, there is perhaps no other researcher who has studied the details of Mars surface mineralogy whose impact was so large, knowledge so broad, and opinions so well respected.
The answer to the question "Why is Mars red?" is that sunlight incident on the surface is preferentially absorbed from the ultraviolet to about 0.6 um via a series of ligand field and cation pair transitions in ferric iron-bearing minerals (e.g., SHERMAN et al., 1982; BELL et al., 1990a). This is well understood, although important debates continue about what specific ferric minerals are responsible for these absorption features. The statement "Mars is red" applies to the planet as a whole, and usually viewed from a large distance. Upon closer examination, a perhaps more interesting question arises: "Why isn't Mars all red?" Or, more specifically, why are some regions of Mars covered by bright, heavily oxidized ferric-rich minerals (SINGER, 1982; BELL et al., 1990a) while many adjacent regions appear to be much less altered mineralogically, containing high abundances of dark, relatively unoxidized ferrous-rich minerals (SINGER et al., 1979; MUSTARD et al., 1993)? I refer to this as the "Mars Weathering Paradox", the solution of which has important implications for understanding the physical and chemical weathering and alteration processes that have operated on Mars.
The abundance, form, and distribution of sulfates and carbonates on the Martian surface also have important implications for past weathering environments, the most important of which may have been acidic groundwaters that chemically altered pristine mafic crustal rocks. The detection of specific hydroxo ferric sulfate and bisulfate ions, Fe-, Al- and Mg-bearing sulfate minerals, bicarbonate ions, carbonate minerals, and clay silicates could provide key indicators of the past and present pH of Martian soils and of the efficiency of chemical weathering under current Martian climatic conditions (BURNS, 1987).
Finally, it has been known since the discovery of the strong 3-um absorption feature in Mars spectra that the surface minerals contain OH and/or bound H2O (e.g., HOUCK et al., 1973). The specific abundance and mineralogy of the OH/H2O-bearing phase(s) has remained uncertain, however. The importance of this issue in terms of surface weathering and alteration is similar to the importance of water in terrestrial weathering processes: water acts as a catalyst to substantially speed chemical reactions on mineral surfaces because reactions involving aqueous phases proceed orders of magnitude faster than gas-solid phase reactions (GOODING and KEIL, 1978; BURNS and FISHER, 1993). Other factors like temperature and pH also strongly influence chemical reaction rates on Mars (BURNS, 1993), as they do on Earth.
In this paper, I review the currently-available spectroscopic data on the iron, sulfate, carbonate, and OH-bearing mineralogy of the Martian surface, present some new observational results, and discuss some new models of surface weathering processes that are consistent with the available data. Particular emphasis is placed on current and past attempts to identify the types of minerals that Roger Burns first realized were so important for untangling complex and often non-unique surface weathering processes. For clarity, in Table 1 I define many of the specific weathering and mineralogical terms used throughout this paper.
Background
The most spectrally-active cation with relevance to Mars is iron. Fortunately, the Martian crust and mantle have relatively high iron contents (e.g., TOULMIN et al., 1977; DREIBUS and WäNKE, 1985), and so Fe-bearing minerals and alteration products exist in enough abundance to be detectable using remote sensing techniques. Almost all of the absorption features that have been definitively detected on Mars and identified with specific mineralogies are due to electronic transitions within Fe3+ cations and charge transfers between these cations and O2- anions (e.g., SHERMAN et al., 1982; SHERMAN and WAITE, 1985; MORRIS et al., 1985), or to electronic transitions within Fe2+ cations and charge transfers between these cations and Fe3+ (e.g., BURNS, 1970; ADAMS, 1974).
Spectroscopic observations
Many telescopic observations of Mars have been performed during this century with the explicit goal of identifying and quantifying the surface and airborne dust Fe mineralogy (for reviews of the earliest observations, see DE VAUCOULEURS, 1954; MCCORD and ADAMS, 1969; BELL, 1992). To within a few percent, the overall shape of the mean visible to near-IR spectral reflectance curve of Mars has been known since the late 1960's.
Continued advances in instrumentation and detector design have allowed observers to obtain detailed, higher spectral resolution and spectral sampling measurements of many small, discrete regions of the Martian surface in the visible to near-IR. Parallel advances in laboratory spectroscopic instruments and other sample characterization techniques have also occurred during this time. As the technology has improved, so has the ability to detect subtle spectral absorption features and to assign them to specific Fe-bearing minerals. For example, MCCORD and WESTPHAL (1971) observed 200 km-sized bright and dark regions on Mars during the 1969 opposition and found that both bright and dark regions are quite red, with the strong 0.4-0.7 um absorption edge having a steeper slope for brighter regions. This has been interpreted as indicating an enrichment in ferric (Fe3+) minerals in the bright areas (e.g., ADAMS and MCCORD, 1969; BINDER and JONES, 1972). Also, a broad and weak absorption band from 0.9-1.1 um was observed in dark region spectra. This band was ascribed to ferrous (Fe2+) cations occurring in silicate minerals such as olivines and pyroxenes (e.g., ADAMS, 1968; ADAMS and MCCORD, 1969; BINDER and JONES, 1972), and the correlation of the strength of this feature with low albedo areas led to the general impression that the dark regions on Mars are more "mafic" in character (with a greater exposure of basalts or other bedrock materials) than the more weathered and altered bright regions. Fig. 1 presents some examples of typical Mars bright and dark region spectra obtained by MCCORD and WESTPHAL (1971) and others.
More recent telescopic and spacecraft observations and re-interpretations of previous data sets have concentrated on providing more specific information on band centers, band depths, and the spatial distribution of observed spectral features, in order to constrain further the surface mineralogy (detailed reviews can be found in SODERBLOM, 1992 and ROUSH et al., 1993). For example, MORRIS et al. (1989) re-examined spectra obtained by MCCORD et al. (1982a) and found that the 0.84-0.90 um band seen in bright region spectra is most likely caused by crystalline hematite ([[alpha]]-Fe2O3) on the Martian surface, mixed with extremely fine-grained or nanophase ferric oxide particles. BELL et al. (1990a) confirmed the existence of hematite in a large range of particle sizes on Mars and placed an upper limit (~ 4%) on the abundance of well-crystalline hematite in the bright region spectra, although the existence of other ferric-bearing minerals with only subtly differing spectral properties could not be ruled out. Differences in the hematite abundance and distribution between and among bright and dark regions have been reported by SINGER et al. (1990), BELL et al. (1990b), and BELL (1992) in high resolution imaging spectroscopic observations of Mars.
Other researchers have interpreted several of these previous data sets as providing evidence of different Fe3+-bearing phases. For example, BISHOP et al. (1993) noted 0.4 to 1.0 um spectral similarities between Mars spectra and a suite of ferrihydrite-bearing smectite clays. BANIN et al. (1993) have presented and reviewed evidence based on 0.4 to 1.0 um spectroscopy and geochemical weathering constraints that lepidocrocite-bearing smectite clays are consistent with the available Mars spectra.
The more recent data sets also confirmed the existence of the weak 0.9-1.0 um Fe2+ absorption feature reported by MCCORD and WESTPHAL (1971) and others. This feature consistently appears deeper in spectra of low albedo regions, and comparisons with laboratory reflectance studies have suggested that this band is due predominantly to low-Ca pyroxene (e.g., SINGER et al., 1979). No firm evidence has yet been found to support the claim by HUGUENIN (1987) of more olivine-rich spectral units on the Martian surface.
An important source of new spectroscopic data was provided by the Phobos-2 ISM instrument, which was an imaging spectrometer that obtained 0.8 to 3.2 um data in 1989 for a part of the Mars equatorial region at ~ 22 km/pixel spatial resolution. MURCHIE et al. (1993) have concentrated on the analysis of bright region mineralogy from the ISM spectra, and have found broad general agreement with previous groundbased studies. These relatively high-spatial-resolution data show clear evidence for crystalline hematite in the bright regions examined, and also show distinct absorptions in a few intermediate-albedo regions that resemble those due to ferric oxyhydroxide phases such as goethite ([[alpha]]-FeOOH) (Fig. 2a and Fig. 2b). This interpretation is also supported by the GEISSLER et al. (1993) analysis of ISM spectra in the Valles Marineris region. They found that spectra of isolated regions in the canyon system show greatly enhanced ferric absorptions suggesting a combination of hematite possibly with a hydrous ferric oxide such as goethite, and this observation along with the presence of associated canyon structural features may imply the existence of a past hydrothermal alteration zone on this part of Mars. MUSTARD et al. (1993) analyzed the ISM spectra with an emphasis on the ferrous mineralogy of Mars and found conclusive evidence for calcic pyroxene in dark region spectra, as well as for subtle variations in pyroxene chemistry from region to region (Fig. 3a). MUSTARD and SUNSHINE (1995) have expanded on this analysis and found that many of the volcanics are two-pyroxene basalts (high- and low-Ca, likely pigeonite and augite) analogous to basaltic compositions found in several of the SNC meteorites (see below), and that the modal mineralogy of these pyroxenes varies across the surface (Fig. 3b Part 1 and Fig. 3b Part 2).
Newer Mars imaging spectroscopic observations were obtained from the Mauna Kea Observatory 2.24-m telescope in November 1990. These spectra cover the 0.50 to 0.95 um region at a spectral resolution of R=200 to 350, and were obtained in order to address the issue of Mars surface ferric and ferrous mineralogy for regions of the planet not observed during the 1986 and 1988 oppositions. Poor atmospheric seeing conditions during the observing run severely limited the resolution of the images to worse than 750 km/pixel (cf. Fig. 4a), but useful mineralogic information can still be extracted by treating the image cubes as a traditional point-spectroscopy data set. However, the effective spatial resolution of these spectra is much lower than was obtained in 1986 and 1988 because the poor atmospheric seeing conditions tend to blur regions together and reduce the ability of detecting subtle spectral differences. Details of the characteristics, reduction, and calibration of these data are presented in the Appendix.
Figs. 4b,c shows some representative spectra from a wide range of regions of different albedo, topography, latitude, and geologic setting that were observed. Several interesting features are seen in these new spectra. First, both bright and dark regions exhibit the characteristic red visible spectral slope typical of Mars. Second, inflections or absorption features are evident near 0.6 to 0.7 um and 0.8 to 0.9 um in both types of spectra. The longer wavelength band has a minimum near 0.867 um (derived using a 2nd order polynomial fit) in the bright regions and near 0.893 um in the dark regions. And finally, the dark region spectra exhibit a more negative spectral slope in the 0.75 to 0.94 um region than the bright regions.
These spectra and others among the many thousands in this new data set show that absorption band positions in most bright regions indicate the presence of a small amount (less than 5%) of well-crystalline hematite that must occur in a matrix of much more poorly crystalline ferric-bearing materials which account for the overall color of Mars (MORRIS et al., 1989; BELL et al., 1990a). The slight shifts in band position observed among and between bright and dark regions and the negative 0.75 to 0.94 um spectral slope are most likely caused by the presence of pyroxene-bearing materials in the dark regions that have ferrous absorption band positions longward of 0.9 um. Alternately, the band shift may be partly due to the presence of hydrous ferric oxides like goethite. Unfortunately, the spectra do not extend to long enough wavelength to unambiguously resolve this question. The position of the near-UV absorption edge, position of the 0.86 um band minimum, and the decreasing near-IR spectral slope argue that band shifts between regions may be indicating slight differences in mixing between hematite-rich dust and soils and pyroxene-rich volcanics at spatial scales smaller than the resolution of the data (e.g., MUSTARD AND BELL, 1994; MORRIS et al., 1995).
Because these data cover a hemisphere dominated by bright, windblown materials and their spatial resolution was poor, there is certain to be a high amount of spatial mixing occurring in the data, such that the spectral differences between regions are muted and even the darkest spectra measured have a substantial "bright" component to them. The only solution to this common problem is increased spatial resolution, utilizing improvements in groundbased techniques, spaceborne observatories, or Mars orbiting or lander spacecraft. Initial tests of this hypothesis that compared Viking Orbiter and Lander color data and Phobos-2 ISM and groundbased spectroscopy have confirmed the importance of spatial resolution in understanding the mineralogy of the Martian surface (e.g., ARVIDSON et al., 1989; MUSTARD AND BELL, 1994). All of these approaches are currently being attempted by many different groups, greatly improving our understanding of Mars spectral heterogeneity.
It has become generally apparent that increases in spectral and spatial resolution and wavelength coverage of Mars observations are necessary in order to identify the surface mineralogy. Higher spectral resolution is needed in order to accurately quantify and map the subtle band shifts associated with the rather weak (typically 1 to 10%) ferric and ferrous bands seen in the data. Higher spatial resolution is needed in order to increase the detectability of minerals that have only a limited spatial extent and whose signature is muted in spectra of regions hundreds to thousands of km in size (e.g., MUSTARD and BELL, 1994). Greater wavelength coverage, encompassing both visible and NIR wavelengths, is critical for discriminating ferric and ferrous minerals having only subtle differences in absorption band shape and position from each other. Thus, while there is no debating the value of high spatial resolution Mariner and Viking Orbiter/Lander imaging of Mars for geologic unit mapping and photogeologic studies, these spacecraft observations have not been optimal for determining the surface mineralogy because of their low spectral resolution, coarse spectral sampling, and limited wavelength coverage.
Geochemical, magnetic, and other data
Much of what we have learned about the Fe mineralogy of Mars comes from telescopic and spacecraft reflectance spectroscopic observations. However, the Viking Landers obtained additional diagnostic information on the bulk chemical composition of the Martian soil and on its optical and magnetic properties. Table 3 presents a summary of the average chemical composition of the Martian soil at the two landing sites from the X-Ray Fluorescence (XRF) analyses of CLARK et al. (1982). All of the soils sampled exhibited high iron contents (ranging from 16% to 19% Fe as Fe2O3). The soil composition was interpreted using normative calculations and compositional mixing models as secondary weathering products of mafic igneous rocks, possibly resulting from palagonitization. In particular, the models yielding the best fits to the data included iron-bearing smectite (nontronite) and/or iron oxides (hematite, maghemite, magnetite) as the major iron-bearing minerals (TOULMIN et al., 1977). The Viking Landers also revealed that the soils are highly magnetic, and HARGRAVES et al. (1977) interpreted the magnetism as due to 1% to 7% maghemite ([[gamma]]-Fe2O3) dispersed as a pigment throughout the surface particles. Estimates of the light scattering and other properties of the Martian airborne dust were obtained by POLLACK et al. (1977) using Viking Lander sky images. They found that the best match to their derived imaginary index values for the dust came from fine-grained magnetite (Fe2+Fe+O4), which could also satisfy the magnetic properties results. It is possible that both of these results are correct, and that both of these iron oxides occur in the Martian soils. The surface magnetic interpretations incorporate not only the bulk magnetic behavior results but also the observed surface color, which is more consistent with maghemite than magnetite; the airborne dust optical properties results are more sensitive to materials with uniformly high absorption coefficients like magnetite, rather than generally less-absorbing minerals like maghemite. Finally, ADAMS et al. (1986) and GUINNESS et al. (1987) analyzed Viking Lander multicolor reflectance data and noted that most of the soils are redder than most palagonites, suggesting that the soils may have a greater degree of ferric iron crystallinity. Dark, gray blocks were found to exhibit colors similar to mafic rocks covered by varying amounts of ferric dust. These authors concluded that the ferric-rich soils observed by the Viking Landers have not been derived from local weathering of the mafic blocks, but more likely have been deposited by aeolian processes such as global dust storms.
Summary: Fe mineralogy
Only two iron-bearing mineral phases have been definitively identified spectroscopically on Mars: hematite and pyroxene (low- and high-Ca). Pyroxene-bearing volcanics are an abundant component of many dark regions, and hematite-rich soil/dust is found in varying levels of abundance and crystallinity in regions of all albedos. Tentative and potentially important spectroscopic evidence for the existence of ferric oxyhydroxide minerals, such as goethite, has been presented for a few isolated intermediate-albedo regions. No firm spectroscopic evidence for other ferric oxide phases exists, although magnetic studies of the Mars soil by the Viking Landers indicate the possible presence of maghemite, and studies of the optical properties of Mars atmospheric dust reveal many similarities with magnetite. Only indirect evidence exists for iron-bearing smectite clay (nontronite), based on modeling of the Viking compositional data. Evidence for other ferrous-bearing phases, such as olivine, has been only circumstantial to date.
Theoretical and terrestrial analog considerations
There is abundant geomorphic evidence that water once flowed across the surface of Mars, suggesting that perhaps the climate was substantially different at some time in the past. In particular, many researchers believe that early Mars went through a warmer and wetter climatic period that may have included a thicker greenhouse atmosphere and/or a higher geothermal heat flux (e.g., POLLACK et al., 1987; FANALE et al., 1992; SQUYRES and KASTING, 1994). If greenhouse warming was important, then many of the atmospheric constituents responsible (CO2, SO2, NH3, CH4, or H2O; see FANALE et al., 1992) have since been lost. One possible sink for the most important and relevant greenhouse gases for Mars (CO2 and volcanically-outgassed SO2 and H2O) is the regolith. For example, on Earth, CO2 is continually being recycled through the crust-atmosphere system in the form of carbonate rocks. This process occurs when CO2 is dissolved in solution, forming an acid which leaches cations like Mg and Ca from silicate rocks. Bicarbonate anions (HCO) are also formed in this process, and the recombination of the Mg and Ca with the HCOcomplexes in aqueous solution leads to the formation of carbonate minerals like dolomite and calcite (e.g., FANALE et al., 1992; SCHAEFER, 1993). A similar process could also occur if atmospheric SO2 was dissolved in solution, forming bisulfate anionic complexes (HSO) and possibly leading to the formation of sulfates. Yet another possible method of sulfate formation is the weathering of surface minerals by sulfuric acid contained in volcanic sulfate aerosol particles (SETTLE, 1979).
Additional theoretical support for the existence of carbonates and sulfates on Mars comes from thermodynamic calculations of the most stable gas-solid decomposition products of major mafic and ultramafic minerals under Marslike environmental conditions. O'CONNOR (1968), GOODING (1978), and GOODING et al. (1992) found that even if Mars did not have a warmer and wetter past climate, gas-solid weathering of olivine, pyroxene, and plagioclase could lead to the formation of stable or metastable carbonate phases like magnesite or siderite. These thermodynamic calculations also indicated that the weathering of igneous sulfide minerals by either gas-solid or liquid-solid mechanisms should lead to the formation of sulfates like FeSO4 and possibly CaSO4 and MgSO4.
On Earth, most chemical weathering processes are cyclical because of the extensive hydrosphere and the role of plate tectonics in recycling crustal materials. On Mars, Earth-like plate tectonics is absent, and the extent and duration of the Martian hydrosphere is highly uncertain, though almost certainly less pervasive than the Earth's. It is also clear that if the Martian climate was warmer and wetter, this period occurred long ago and the climate has been more similar to its present state for at least several billion years (e.g., SQUYRES AND KASTING, 1994). These factors argue that for Mars the formation of carbonates or sulfates from an early dense atmosphere or by gas-solid weathering may have been a one-way trip, and that the current surface and crustal mineral inventory may still sequester a substantial abundance of CO2 and SO2. This possibility has spurred substantial interest in searching for quantitative evidence of carbonate and sulfate minerals on Mars.
A recent complication has arisen from the calculations of KASTING (1992), who has found that condensation of CO2 clouds in a dense early Mars atmosphere (and assuming a fainter young Sun) would substantially reduce the magnitude of the greenhouse effect. Thus, attention has returned to the need for alternate trace greenhouse gases as sources of heat for early Mars, with SO2 being perhaps the most plausible gas, as it is a common outgassing product of terrestrial volcanism (e.g., POSTAWKO and KUHN, 1986). CH4 and NH3 are also good greenhouse gases, but they are photochemically unstable in the current high-UV flux environment of the Martian atmosphere (KUHN and ATREYA, 1979), so they would not be able to influence atmospheric heating on long timescales unless there were a constant and abundant resupply of these gases over geologic time.
If SO2 was more abundant in the early Mars atmosphere, then its aqueous dissolution and the formation of HSOcould have played an important role in chemical weathering of surface igneous silicates and sulfides by leaching out Fe, Mg, and Ca and forming sulfates and other silicates from solution. Alternately, sulfur could have segregated from magma at depth to form massive iron sulfide deposits, and the interaction of groundwater with these deposits would yield acidic solutions capable of weathering the surrounding rock. BURNS (1987, 1988) invoked such a weathering process to explain the high S abundance observed by the Viking Landers, and also showed that spectra of the Martian surface in the visible and near-IR are consistent with spectra of ferric-bearing sulfate phases (like jarosite). According to BURNS (1987), a byproduct of this aqueous weathering process, dependent on pH, would be the formation of poorly crystalline iron oxides and clay silicates which, after desiccation, could also yield fine-grained and highly magnetic phases like maghemite ([[gamma]]-Fe2O3) in the soils. Most recently, MORRIS et al. (1996) have provided an example of the formation of sulfates (jarosite in this case) by a different process with relevance to Mars, involving the action of S-rich volcanic gases and the subsequent hydrothermal alteration of tephra by acidic solutions.
Viking Lander and SNC meteorite measurements
One of the biggest surprises from the Viking XRF measurements was the discovery of a much higher than expected abundance of sulfur in the soils at both landing sites (Table 2). TOULMIN et al. (1977) interpreted the high abundance of sulfur as sulfates occurring in a surface duricrust, responsible for the hardpan or cemented appearance of some regions around the landers (Fig. 5a). The apparently oxidizing nature of the soils argued that the sulfur occurs in sulfates rather than in reduced forms like pyrrhotite (Fe1-xS), thus ruling out the possibility that the sulfur-bearing phases could be responsible for the observed soil magnetism.
Evidence for carbonates in the Martian soils from the Viking Lander data has only been indirectly provided. BIEMANN et al. (1977) reported that CO2 was evolved from the surface soils in the Viking GCMS experiment, and TOULMIN et al. (1977) use this result and thermodynamic stability arguments to conclude that CO2 may represent a significant portion, second only to H2O, of the "missing" (undetectable) chemical components in the soil (Table 2). In their normative mineralogy and mixing calculations, TOULMIN et al. (1977) favored a model having from 7% to 9% calcite in the surface soils as consistent with the Viking XRF data. However, simulations of the Viking Pyrolitic Release (PR) and Labeled Release (LR) biology experiments (e.g., HUBBARD, 1979; BANIN AND RISHPON, 1979) demonstrated that inclusion of more than 0.5 to 1.0% calcite, magnesite, or dolomite results in a substantial reduction of the release of 14CO2 and is not consistent with the Viking data. These authors found that a much higher concentration of siderite could exist and still be consistent with the Viking data, though, so the interpretation of the PR and LR results (as well as the XRF data) in terms of carbonates is still somewhat uncertain.
Recent detailed petrographic analyses of the SNC (Shergottites, Nakhlites, and Chassigny) meteorites, thought to be samples of the Martian crust and mantle delivered to Earth by impact(s) on Mars, have revealed traces of water-precipitated minerals, including carbonates and sulfates (e.g., GOODING and MUENOW, 1986; GOODING, 1992; MCSWEEN, 1994). These minerals provide evidence for regolith weathering products similar to what have been theoretically predicted. Isotopic analysis of the SNC carbonates has been used to show that the weathering took place at low temperature (CLAYTON and MAYEDA, 1988). The nonuniform distribution of these low-temperature weathering products within the meteorites and the chemical similarities between some of the weathered minerals and their precursor phases argues that the weathering occurred during episodic infiltration of small volume of saline water (TREIMAN et al., 1993; MCSWEEN, 1994), rather than during a steady-state aqueous environment.
Spectroscopic observations
There have been many spectroscopic searches for sulfate and carbonate minerals on Mars in order to try to quantitatively substantiate the theoretical and indirect arguments discussed above for the occurrence of these phases. Accurate detection and identification of these minerals requires observations in the near-IR to mid-IR, where the strongest vibrational fundamental and overtone bands from CO, HCO4- , SO, and HSO4- anions occur (e.g., BURNS, 1970; HUNT and SALISBURY, 1971; SWAYZE and CLARK, 1990; GAFFEY et al., 1993). Thus, many of the highest-quality data sets with which to assess the presence or absence of sulfate and carbonate minerals have been obtained only recently and have coincided with advances in modern infrared detector technologies. Important early exceptions are: (1) the Mariner 6 and 7 Infrared Spectrometer (IRS) data set, which, although more than 25 years old, continues to remain unique in terms of spatial and spectral coverage among telescopic and spacecraft investigations of Mars. ROUSH et al. (1986) and MCKAY and NEDELL (1988) searched through the IRS data set for evidence of anhydrous carbonate absorption features, but found nothing conclusive. And (2) the Mariner 9 infrared interferometer-spectrometer (IRIS) data set, obtained in 1971 and which continues to be the primary source of high-quality thermal emission spectra of Mars. HUNT et al. (1973) and TOON et al. (1977) searched through the IRIS data set for evidence of carbonates, but were only able to place broad upper limits on the abundance of carbonate minerals in the global dust (a few to 10%).
More recent spectroscopic searches for evaporite minerals on Mars have been conducted by several groups. BLANEY and MCCORD (1989a) observed large regions of the planet in the 2.5 to 4.2 um region and provided upper limits on the possible presence of carbonates based on the detection of weak spectral features near 4 um. Subsequent observations reported by BLANEY and MCCORD (1989b) also detected these weak spectral features, and they were interpreted at the time as possibly originating from H-S stretch absorption features in hydroxy-acid salts or other sulfates. However, modeling by ENCRENAZ and LELLOUCH (1990) demonstrated that much of the spectral structure detected near 4 um could be attributed to isotopically heavy CO2 in the Martian atmosphere. BLANEY AND MCCORD (1995) extended their observations into the 4 to 5 um region and detected an absorption feature near 4.5 um that was interpreted as an SO42- overtone band in sulfates in the Martian surface and/or airborne dust. Careful atmospheric modeling revealed that this feature is likely not due to any known species in the Martian atmosphere; however, the specific mineralogy responsible was not identified because of the inherent difficulties of analyzing a spectral feature that occurs on the wing of the strong 4.2-4.4 um Mars atmospheric CO2 band. POLLACK et al. (1990) used the Kuiper Airborne Observatory (KAO) to obtain high-quality spectra in important wavelength regions that cannot be observed from groundbased telescopes. These data showed evidence for spectral features due to carbonate (near 6.7 um) and sulfate (near 8.7 and 9.8 um) anionic complexes in the airborne dust, but specific mineralogies could not be identified. CLARK et al. (1990) observed Mars in the 2.0 to 2.5 um region and reported bicarbonate and/or bisulfate absorption features with positions and relative intensities that they claimed were diagnostic of the mineral scapolite. However, the occurrence on Mars of large quantities of this rather rare metamorphic mineral was met with substantial skepticism by geochemists and others. BELL et al. (1994a) obtained new spectra in this wavelength region and showed that several of the spectral features reported by CLARK et al. (1990) and others were probably due to residual telluric, Martian, or solar atmospheric bands. The remaining mineralogic features could still be caused by (bi)carbonate or (bi)sulfate complexes, although assignment of a specific mineralogy was not possible using the available data. Finally, CALVIN et al. (1994) have used a combination of groundbased spectra and a re-analysis of the Mariner IRS data to infer the existence of hydrous carbonates on Mars. Hydrated carbonates such as hydromagnesite [Mg5(CO3)4(OH)2 * 4H2O] show much weaker spectral features than their anhydrous counterparts, and the positions of some of the weak absorption bands seen in terrestrial samples was found to match the positions of certain features seen in telescopic and Mariner IRS spectra of Mars. Hydrous carbonates represent a double-edged sword of sorts because they may occur on Mars in enough quantity to store a substantial reservoir of CO2, but they may not be detectable spectroscopically even at the tens of percent level.
Summary: Sulfates and carbonates
Compelling theoretical arguments suggest that Mars once had a denser atmosphere, and strong evidence for this theory would be provided by the discovery of a global inventory of carbonate or sulfate minerals that have sequestered a large fraction of important greenhouse gases. A high sulfur abundance was measured in the Martian soils by the Viking Landers, but the specific sulfate mineralogy was not identified by Viking nor has it been well-constrained by groundbased or spacecraft spectroscopic observations since then. Carbonate minerals were also inferred based on the Viking Lander measurements. The SNC meteorites, thought to be samples of the Mars crust and/or mantle, have been found to contain small amounts of pre-terrestrial carbonates and sulfates. Groundbased and spacecraft searches for anhydrous carbonates have not conclusively identified any specific minerals in the uppermost few microns of the Martian surface, but evidence for (bi)carbonate and (bi)sulfate anionic complexes has been found in several spectroscopic data sets.
Background
The distribution and abundance of water in the surface rocks and soils of Mars has important implications for the style and rate of chemical weathering, for surface/atmosphere volatile transport, for the history of volatile outgassing, and for exobiology. The low temperatures and water contents of the current Martian environment place severe constraints on weathering rates, to the point that mineral phases predicted to be thermodynamically unstable on Mars (e.g., O'CONNOR, 1968; GOODING, 1978) may actually persist over geologic timescales. For example, BURNS (1993) postulated that (partially) dehydroxylated clays may have formed earlier in Martian history and remained metastable under current atmospheric conditions. Thus, observations of relict water- or hydroxyl-bearing minerals which are currently thermodynamically unstable at the surface could provide a way to quantify previous climatic regimes, assuming that the detected phases formed under equilibrium conditions with an earlier atmosphere.
Spectroscopic observations
Spectroscopic detection of adsorbed or structurally-bound water or OH in minerals is achieved by observations of rather broad features at many of the same wavelengths as the fundamental and overtone/combination bands of water vapor (e.g., HUNT et al., 1971; GAFFEY et al., 1993; BISHOP and PIETERS, 1995). Thus, it is difficult to detect unambiguously these features from groundbased telescopes observing Mars through the Earth's water-rich atmosphere. Nonetheless, SINTON (1967) provided the first indication of bound water on Mars by observing indications of a depression in the spectrum near 3 um (the O-H stretching vibrational fundamental). Additional indications of continuum absorption ascribed to bound water was provided by the high-resolution spectra of BEER et al. (1971). However, the most convincing measurements were those obtained by HOUCK et al. (1973) using an airborne telescope at 12 km altitude, above most of the Earth's water vapor. These authors observed a broad, ~80% deep absorption band centered near 2.85 um, and their modeling results indicated that the fraction of bound water and/or OH- in the surface materials is ~ 1%.
These groundbased and airborne results were verified by analysis of the Mariner 6 and 7 IRS spectra of Mars (PIMENTEL et al., 1974). The IRS data show a strong 2.85 to 3.30 um absorption band that varies in strength from region to region on Mars. Various spectral ratios and correlations with other parameters (albedo, latitude, etc.) were used to discriminate between hydrated minerals and water ice as the cause of the 3-um absorption. Water (and CO2) ice absorptions were found in the IRS data over the polar regions, but at other latitudes the 3-um band is entirely consistent with the O-H stretching vibrational overtone observed in many hydrated silicate minerals.
The strong 3-um hydrated mineral feature has been observed more recently from groundbased telescopes and from the Phobos-2 ISM spectrometer, which have revealed more details on the spatial distribution of the 3-um absorption. BELL and CRISP (1993) found spatial variations in the strength of the 3-um absorption in equatorial and mid-latitudes that is correlated with albedo (Fig. 6). MURCHIE et al. (1993) also found that the 3-um band strength is generally greater in high albedo regions than in low albedo regions. However, there are discrete areas exhibiting enhanced 3-um absorptions, including layered deposits in Valles Marineris and (perhaps not coincidentally) the regions having Fe absorptions resembling those of ferric oxyhydroxides. Their ISM data revealed two classes of soils based on 3-um band strength: "normal soils" with a band typically 50 to 60% deep, and less common "hydrated bright soils" with a band several percent deeper than in other materials of comparable albedo. MURCHIE et al. (1993) inferred that variations in soil hydrated mineral lithology offered the most likely explanation for these 3-um band variations.
The 3-um hydrated mineral feature indicates that a substantial amount of water is tied up in the rocks and minerals on the Martian surface (20 pr um in the upper few mm as estimated by HOUCK et al., 1973). However, the specific minerals responsible for this spectral feature have not been unambiguously identified because the 3-um feature does not exhibit very sensitive spectral variations with changes in mineralogy. Other features that involve both adsorbed and structurally-bound water do exhibit diagnostic variations, however. Most notable are absorption features caused by cation-OH stretching vibrational fundamentals, overtones, and combinations in clay silicate minerals. Many of these features occur in the 2.2 to 2.3 um region, which is a transparent window in both the terrestrial and Martian atmospheres. Examples include the 2.2 um Al-OH feature in montmorillonite and the 2.3 um Fe-OH feature in nontronite. However, even in this wavelength range the Martian atmosphere contains absorptions due to CO2 and CO that complicate the identification and assignment of absorptions from surface minerals.
Spectroscopic searches for cation-OH and OH- overtone and combination features have been conducted by many researchers, often with conflicting results. Data obtained and analyzed by MCCORD et al. (1978, 1982a) showed a complex absorption feature near 1.95 um that was interpreted as being composed of several lattice vibrational modes of clay minerals like montmorillonite and kaolinite. Small, distinct absorption bands in the 2.2-2.5 um region can also be seen in their spectra. These authors and SINGER et al. (1979) attribute weak features near 2.3 um and possibly 2.4 um to Mg-OH in sheet silicates (serpentine, talc, Mg-smectites) or amphiboles (anthophyllite, actinolite). They interpret the lack of a 2.2 um feature (characteristic of Al-OH) as evidence that montmorillonite or other dioctohedral clays are not a major component of the Martian regolith. Further study by SINGER (1982) concluded that nontronite is not a major component of the Martian soil since its visible reflectance signature is unlike that of the available data. However, the presence of other iron-poor clays (such as montmorillonite) could not be totally excluded.
As discussed previously, narrow features in the 2.1 to 2.5 um spectra of CLARK et al. (1990) have been interpreted as due to carbonate- or sulfate-bearing minerals, as opposed to clay or other hydrated silicates. BELL and CRISP (1993) noted evidence for absorption features near 2.25 um in their imaging spectroscopic observations of Mars, and speculated on their possible origin as Fe-OH overtones. MURCHIE et al. (1993) also observed a weak absorption from 2.20 to 2.25 um in ISM spectra covering bright regions, and interpreted this feature as consistent with a mixture of poorly-crystalline phyllosilicates (Fig. 2a and Fig. 2b). As with the 3-um H2O absorption there is a general correlation of band strength with albedo, but discrete high-albedo features (layered deposits in Candor Chasma) lack this absorption. This spatial coherence of variations in band strength with geology is perhaps the strongest evidence from ISM data for association of this feature with surface mineralogy. BELL et al. (1994a) were able to confirm that at least some of the narrow, weak features seen in Mars spectra in the 2.0 to 2.5 um region are due to surface mineralogy (with the most likely origin of these features being either (bi)carbonate or (bi)sulfate anions in framework silicates or (Fe, Mg)-OH bonds in sheet silicates), while other features appear to originate from telluric, Martian, or solar atmospheric absorptions.
The KAO mid-IR spectra of POLLACK et al. (1990) discussed above showed evidence for an emission maximum near 6.2 um that was attributed to hydrated surface minerals, but the specific mineralogy could not be identified. Spectroscopic observations by the Mariner 9 IRIS instrument detected spectral features near 9.0 um in the atmospheric dust that were interpreted to be most consistent with montmorillonite (HUNT et al., 1973) or a basalt-montmorillonite mixture (TOON et al., 1977). More recent re-analyses of the IRIS measurements have shown that poorly-crystalline weathering products like palagonite may in fact be more consistent with the data than smectite clay, however, based on improved knowledge of the optical constants of palagonites and on the scattering properties of atmospheric dust particles (ROUSH et al., 1993; CLANCY et al., 1995).
A perplexing observation, noted by BELL et al. (1994a), is that many of the observed spectral features on Mars that occur at or near wavelengths characteristic of OH-bearing minerals are much narrower than their counterpart bands in terrestrial samples. A possible explanation was proposed, based on the dehydroxylated clays study of BURNS (1993): one effect of dehydroxylation could be to reduce the strengths and possibly, under certain circumstances, the widths of both OH- and cation-OH absorption features in the near-infrared. However, evidence for changes in the nature of structural OH absorption features with variations in pressure and temperature appropriate for the Martian environment were not found by BISHOP and PIETERS (1995) in their analysis of Mars soils analog materials. These authors did find evidence for systematic changes in the depth and width of H2O stretching and bending combination bands in the near-IR with changes in environmental conditions and with variations in the nature of interlayer smectite anionic complexes. An alternate explanation for the narrow band widths, frustrating for Earth-bound mineralogists, is that a continuum of crystalline and amorphous salts, clays, and/or clay-like phases may exist on Mars that simply do not have good terrestrial analogs.
Other sources of information
As mentioned above, although the Viking Landers did not directly determine surface mineralogy, the chemical composition results of the XRF experiment have been used to construct mixing models of possible surface mineralogies. The best-fit models included nontronite and montmorillonite as prominent surface minerals, in abundances around 50% and 20%, respectively (TOULMIN et al., 1977). However, these models are based on normative mineralogy calculations, and make certain geochemical assumptions that may not necessarily be unique or appropriate for the Martian surface. BANIN and RISHPON (1979) and BANIN and MARGULIES (1983) in their simulations of the Viking Lander LR experiment concluded that smectites, but not the smectite-poor palagonites that they examined, could reproduce the LR data. In particular, Fe-bearing montmorillonite and nontronite were found as the best-fit smectites, while Na- or Ca-bearing smectites did not fit as well. Thus, it is clear that the available data are consistent with the presence of a significant iron-bearing clay silicate component in the Martian soils; however, to date such minerals have not been directly detected or measured either by in situ analyses or remote sensing.
Analyses of SNC meteorites have also detected evidence of presumably Martian smectites, most notably the deposits of "iddingsite" (ferrihydrite + smectite clay) detected in the Lafayette meteorite by TREIMAN et al. (1993), and the possible identification of illite and smectites in EETA79001 and Nakhla (GOODING and MUENOW, 1986; GOODING, 1992). GOODING (1992) found that a mixture of these two phyllosilicate assemblages provides a close match to the elemental abundances measured by the Viking XRF experiment, and MURCHIE et al. (1993) noted that such a mixture would also yield a metal-OH overtone having a wavelength position similar to that found by them and BELL AND CRISP (1993).
Finally, observations of water vapor in the Martian atmosphere as a function of time by the Viking Orbiter Mars Atmospheric Water Vapor Detection (MAWD) experiment obtained results relevant to the study of hydrated minerals on and in the surface (FARMER et al., 1977). These data, acquired over the course of more than a Mars year, showed evidence for the transport of water vapor from the northern to the southern hemisphere during the northern spring and summer, but they did not show any evidence for the return circulation in the northern fall and winter (JAKOSKY and FARMER, 1982). Because MAWD was sensitive only to water vapor and not to hydrated minerals or surface/atmospheric water ice, the current view of what maintains the seasonal hemispheric asymmetry in water vapor or what processes recharge the water reservoirs (regolith and polar cap) each year in the north is obviously incomplete. However, it is likely that the exchange of water between the atmosphere and the surface, through adsorbed and possibly even structural water in minerals, plays an important role. Unfortunately, detailed spectroscopic studies of hydrated minerals as a function of latitude and season on Mars are lacking at the present time.
Summary: Hydrated minerals
A large body of evidence indicates that substantial amounts of adsorbed and/or structurally-bound water exist in the surface minerals of Mars. Identification of the specific hydrated mineral(s) occurring on the surface is equivocal, however, because of the limited diagnostic ability of the O-H and H2O stretching vibrational features in minerals and because of limitations on the quantity and quality of appropriate Mars spectral data. The fact that a large inventory of water appears to be sequestered in the surface minerals is intriguing, and argues that conditions more favorable for aqueous alteration and weathering may have been more common during past epochs when these minerals were originally formed. Although many H2O and OH bearing minerals should not be thermodynamically stable in the current desiccating Martian environment, convincing arguments have been presented indicating that such minerals may in fact persist metastably because current chemical weathering rates are exceedingly slow.
CONSISTENT WITH THE AVAILABLE DATA
The data discussed above allow for the development of a set of consistent and reasonable models of the Mars surface chemical weathering and alteration environment over geologic time. Such models have been discussed in detail for carbonates and sulfates (e.g., FANALE et al., 1982, 1992; SCHAEFER, 1993; BURNS, 1993), as well as hydrated minerals (e.g., SCHAEFER, 1990; GOODING, 1992). Here, I discuss possible scenarios for the weathering and alteration of ferric and ferrous minerals that are consistent with available Mars observations.
Mars, as a whole, appears quite red as viewed by naked eye or by other low spatial resolution techniques. Upon closer examination, such as from high resolution telescopic or spacecraft views, it becomes clear that all regions of Mars are not equally red and oxidized, but that there are substantial variations from region to region (e.g., BELL et al., 1990a,b, 1995; MURCHIE et al., 1993; MUSTARD et al., 1993). These variations generally correlate with albedo (although there are exceptions), with bright regions typically showing the most oxidized spectral character and the dark regions, including many adjacent to the bright regions, typically showing the least oxidized spectral behavior. This is the Mars weathering paradox discussed above, the solution to which has important implications for understanding the physical and chemical weathering and alteration processes that have operated on Mars.
Two possible scenarios for the weathering and/or alteration of Mars surface Fe-bearing minerals are proposed (BELL et al., 1994b):
Decoupled soil and bedrock
In this model the dark, less-red, and relatively pristine surface material such as that observed in Syrtis Major is unweathered pyroxene-bearing bedrock that is covered by or simply mechanically mixed with unrelated ferric materials. Thus, the unweathered rocks are not the source of the bright, ferric-bearing global dust, which must have been formed under different past conditions.
An important question that must be addressed if this model is valid is: Where do the oxidized (ferric) minerals come from? There are at least two possible answers. First, there may have been different climatic conditions in the distant past that allowed much higher weathering rates than at present. Second, individual alteration event(s), such as impacts or volcanism, may have catalyzed the Fe2+ -> Fe3+ conversion, regardless of the climatic regime. The implications are that the present inventory of ferric phases on Mars may be a fossil remnant from a past epoch or past event(s), and that the current distribution of ferric minerals may have little or no genetic relationship to the underlying bedrock. In addition, this model offers the intriguing possibility that the degree of oxidation of dark basaltic materials can be used as a relative age-dating tool on Mars, with dark oxidized regions being older and dark unoxidized regions being younger.
Coupled soil and bedrock
In this model the dark material is weathered or weathering (slowly), creating ferric-bearing surface materials that are mobilized through spallation and erosion. Thus, the bright, ferric-bearing global dust is a direct weathering product of the dark bedrock units, raising the possibility of localized genetic links between weathered and un-weathered materials.
If this model is valid, an important question that must be answered is: Why are the ferrous-bearing surface materials apparently so pristine? One solution is the extremely slow weathering reaction rates in the current environment, which effectively prevent oxidative weathering from occurring at the surface over even geologic timescales (BURNS, 1993). Another possible solution is that the environment conducive to oxidative weathering occurred in association with episodic or sporadic events, none of which have occurred in recent geologic time. The implications of this model are that the basaltic units on Mars are currently weathering, albeit slowly, and that the ferric-bearing dust and soils are a direct product of in situ weathering by surface reactions.
There is evidence supporting both the coupled and decoupled models in currently-available remote sensing data. For example, the presence of well-defined and rather deep Fe2+ (pyroxene)
absorption features in the ISM spectra (e.g., MUSTARD et al., 1993) and evidence from spacecraft and HST imaging for temporal variations in the distribution of bright materials in and around dark regions (e.g., JAMES et al., 1994) argues for the decoupled soil/bedrock model. The alternative, that the basalts are either very coarse-grained or extremely young, are not consistent with other available data, such as thermal inertia and relative age dating from crater statistics. Also, Viking Lander images showing dark, basaltic rocks covered by an apparent sprinkling of bright but presumably genetically unrelated red dust (Fig. 5b; e.g., ADAMS et al., 1986) argue that the soils and bedrock, at least in those regions, are decoupled. The presence of weak or intermediate-position Fe2+ absorption features in some ISM and telescopic dark region spectra provides possible evidence for the coupled model, in that such spectral signatures are consistent with partial weathering of initially more pristine ferrous-bearing materials (e.g., MORRIS et al., 1995). This evidence is not unique, though, as it has been shown that spatial averaging of bright and dark regions as often occurs in low spatial resolution observations of Mars can lead to apparent weakening of Fe2+ band positions (MUSTARD and BELL, 1994). Additional evidence for the coupled soil/bedrock model comes from telescopic, Viking, and ISM observations of ferric-bearing dark regions (e.g., ARVIDSON et al., 1982; MCCORD et al., 1982b; SINGER and ROUSH, 1983; SINGER et al., 1990; FISCHER and PIETERS, 1993; MUSTARD and SUNSHINE, 1995; MURCHIE et al., 1996). Negative visible to near-IR spectral slope and correlations with higher thermal inertia values indicate that the spectra of some of these areas may not represent simple mixtures of ferric-bearing dust and pristine basalt, but instead may represent basaltic regions coated by a ferric stain, somewhat analogous to terrestrial desert varnish.
Discriminating between models
Clearly, the quality and quantity of currently-available remote sensing evidence do not strongly favor one weathering model over the other. In fact, both models may be valid independent of each other in localized regions and/or epochs. The key to solving this ambiguity is to accurately measure the spectrum of the rock "endmember" on a region-by-region basis. This will allow an unmixing of the soil and bedrock phases to be performed and an analysis of whether the bedrock materials are truly unoxidized or whether they are in fact actively oxidizing. Specific observational tests that could be used in this analysis are discussed in the next section.
Continued advances in the study of Mars surface mineralogy are dependent on the ability to obtain higher-quality observations at increased spatial resolution in order to reduce the spectral mixing effects introduced by averaging many regions into a single measurement. It is particularly important to capitalize on the opportunity to observe Mars from groundbased and spaceborne platforms during the upcoming series of favorable oppositions over the next decade and during the current extremely low average atmospheric dust opacity conditions (e.g., Fig. 7). The presence of any amount of dust in the Martian atmosphere acts to effectively degrade the spatial resolution of surface observations and to reduce the detectability of many materials that exhibit less spectral contrast than minerals in the airborne dust. Three specific types of continuing observations are needed:
Visible to near-IR (VNIR) imaging and imaging spectroscopy
VNIR spectra continue to provide the best remote means of identifying the mineralogy of specific alteration products on Mars. As discussed above, spectra at the wavelengths where reflected solar flux is primarily being measured (0.3 to ~3.5 um) are sensitive to variations in ferric iron mineralogy, hydrated minerals including clays, and certain salts and evaporites. In addition, diagnostic information on the pyroxene mineralogy of primary basalts can be obtained from spectra in the 1- and 2-um regions. The key to using VNIR techniques to extend the current knowledge is to apply them in an imaging context, so that regional variations in spectral properties at fine scales can be correlated with similar-scale variations in surface morphology, geology, topography, thermal inertia, etc. For example, it would be extremely interesting to determine whether spectral slope and/or band depth (diagnostic of the degree of surface weathering and alteration) correlates with surface age as determined by other techniques (craters, superposition). The result could be used to assess whether the coupled or decoupled weathering scenarios discussed above are operating in each region. The Phobos-2 ISM data set has effectively demonstrated the value of VNIR imaging spectroscopy for Mars; however, such data need to be obtained over all solar reflectance wavelengths at higher spectral resolution (in order to resolve better narrow absorption features) and over the entire planet at scales of meters to kilometers.
Infrared (IR) thermal emission spectroscopy
IR spectroscopy (~3.5 to 25 um) is much more sensitive to variations in primary silicate mineralogy than VNIR techniques (e.g., FARMER, 1974; SALISBURY et al., 1991). Also, many of the strongest and most diagnostic fundamental spectral features of evaporite minerals occur at thermal wavelengths, making detection and identification of these materials potentially easier in the IR than in the VNIR. The Mars Global Surveyor Thermal Emission Spectrometer (TES) instrument (CHRISTENSEN et al., 1992) will have a much greater ability than previous IR investigations to spectrally characterize the Martian surface, and will do this globally from Mars orbit at several km resolution. In particular, TES will be able to search for any evidence of carbonate or sulfate spectral features in the IR, and to map the distribution of these features. Recent groundbased spectral observations in the IR have also shown dramatic improvements in sensitivity and spatial resolution (e.g., MOERSCH et al., 1993; ROWLAND et al., 1995). Such observations have the potential of substantially enhancing the TES data by observing surface regions at different times of day and different photometric angles. It must also be remembered that groundbased and orbital IR measurements are also observing the highly altered soils and dust, and so a more complete understanding of the spectral properties of poorly-crystalline or highly-oxidized materials in the IR (e.g., ROUSH AND BELL, 1995) will be critical for interpreting Mars thermal IR data sets.
In-Situ analyses
Finally, there are a host of measurements that can be conducted from surface landers or (eventually) trained human explorers that will be able to answer key questions on the iron, sulfur, carbonate, and hydrate mineralogy of Mars. Examples include (1) surface lander VNIR and IR imaging and spectroscopy, able to address the mineralogic issues discussed above but at meter to centimeter spatial resolution; (2) chemical composition experiments such as XRF or DSC/TGA which can determine the abundances of key rock-forming elements or constrain the mineralogy of volatile-bearing phases in the soils at different places; and (3) Mössbauer spectroscopy, which can uniquely and definitively identify many iron-bearing minerals like iron oxides. Many such experiments are currently being proposed by NASA and others as part of planned Mars landing missions over the next decade.
The Martian surface is composed of a complex mixture of rocks and minerals that tell a story about the past history of the atmosphere, hydrosphere, and crust. Fundamental to untangling this story is a more complete knowledge of the current mineralogic inventory of Mars and of formation pathways for secondary minerals, specifically concentrating on the kinds of climatically-diagnostic materials (ferric oxides/oxyhydroxides, carbonates, sulfates, and hydrates) that Roger Burns frequently advocated as the key to understanding the past and current Martian environment.
Our current knowledge is limited to a few specific items:
(a) Most bright surface regions and the airborne dust are highly oxidized and oxidizing, as evidenced by the abundance of Fe3+-bearing minerals like hematite and the results of several of the Viking Lander wet chemistry experiments. Evidence suggesting other (unidentified) iron oxide/oxyhydroxide phases exists from groundbased and spacecraft imaging, spectroscopy, and magnetic experiments, and efforts are being made at determining the spatial distribution of these highly altered phases.
(b) Many dark surface regions are composed of relatively unaltered pyroxene-bearing (Fe2+) basalts. It is unclear why or how these rather pristine, unweathered regions coexist with the highly oxidized areas. Detailed observations and models are beginning to be able to detect variability in the composition of these basalts and to map their spatial distribution.
(c) Abundant geomorphic evidence suggests that water once flowed on Mars. The possible implications for a past warmer, wetter Mars climate have led to intense searches for hydrated mineral phases and evaporites, as these minerals could have sequestered much of the water and greenhouse gases of a putative past thicker atmosphere. To date, tentative evidence of carbonate minerals has been provided by groundbased, airborne, and spacecraft observations, but definitive mineralogic identifications and detailed spatial mapping of these materials is lacking. Evidence for sulfur-bearing minerals comes primarily from Viking XRF measurements of high S in the soils and tentative groundbased detection of sulfate mineral absorption features. Again, however, detailed mineralogic identifications and spatial distributions have not been determined.
There are a number of groundbased, spacecraft, and in situ measurements that can be performed in order to try to remove some of the ambiguity in mineralogic identification and in order to try to correlate the observed mineralogic variations with variations in other physical properties like geology and topography. Collection and interpretation of such new measurements represents a fundamental goal in the continued exploration of Mars.
COLLECTION, REDUCTION, AND CALIBRATION OF 1990 MARS SPECTRAL DATA
Imaging spectroscopic data of Mars were obtained from the University of Hawaii 2.24 m telescope at Mauna Kea Observatory on 1990 November 9 and 10 UT (Table 3). The data were obtained with the Wide Field Grism Spectrograph (WFGS), a facility instrument operated by the Institute for Astronomy at the University of Hawaii. WFGS uses an 800x800 CCD and a transmission grating ruled on a prism to disperse a spectrum along one axis of the CCD array. We used a grating blazed at 4800 Å in first order to obtain data from 0.50 to 0.95 um at a spectral resolution of R = 200 to 350. WFGS is a field widened spectrograph that can accommodate a variety of user-designed slits. Our Moon/Mars slit design had projected dimensions of 0.29" x 153", allowing for high spectral resolution and adequate cross-slit spatial sampling of the Martian disk. Individual images at a number of slit positions on the sky were obtained by sequentially scanning the slit across the disk of Mars. In this way, image cubes were built up by using telescope motion to construct one spatial axis (across-slit) and obtaining individual images in a spatial x spectral format (along slit x dispersed spectrum). A typical Mars image cube consisted of approximately 20x30 spatial pixels at ~ 160 useful wavelengths. Due to rather poor atmospheric seeing conditions during the observing run, the actual image quality corresponded to only 1" to 2", equivalent to regions from 750 to 1500 km in diameter, and thus the imaging aspect of these measurements did not yield much success (Fig 4a).
Eleven image cubes were obtained centered near 100-140deg. W longitude. Mars subtended 17.8" during this time, was at a phase angle of 17deg., and the season was late northern winter (Ls = 330deg.). We also obtained 10 image cubes of the solar analog star [[kappa]] Cetus (Type G5V, MV = +4.82; HARDORP, 1978) and numerous bias and flatfield calibration frames. As an aid in reconstruction of the slit-scan images, we also obtained direct red filter CCD images of Mars. Wavelength calibration was performed using Hg-Cd-Zn and Ne calibration lamps.
Data reduction procedures are similar to those used for 1988 WFGS data described previously by BELL et al. (1990b) and BELL (1992) for different regions of the planet. These procedures include bias and dark current removal, flatfield (pixel-to-pixel non-uniformity) corrections, assembly of the individual slit positions into image cubes, and spatial reconstruction of the imaging data so that the geographic locations of spectra could be determined. We were not able to completely remove the residual effects of telluric O2 absorption near 0.76 um, so the spurious data near that wavelength have been removed.
ADAMS J.B. (1968) Lunar and Martian surfaces: Petrologic significance of absorption bands in the near-infrared. Science 159, 1453-1455.
ADAMS J.B. (1974) Visible and near-infrared diffuse reflectance spectra of pyroxenes as applied to remote sensing of solid objects in the solar system. J. Geophys. Res. 79, 4829-4836.
ADAMS J.B. and MCCORD T.B. (1969) Mars: Interpretation of spectral reflectivity of light and dark regions. J. Geophys. Res. 74, 4851-4856.
ADAMS J.B., SMITH M.O., and JOHNSON P.E. (1986) Spectral mixture modeling: A new analysis of rock and soil types at the Viking Lander I site. J. Geophys. Res. 91, 8098-8112.
ARVIDSON R.E., GUINNESS E.A. and ZENT A.P. (1982) Classification of surface units in the equatorial region of Mars based on Viking Orbiter color, albedo, and thermal data. J. Geophys. Res. 87, 10149-10157.
ARVIDSON R.E., GUINNESS E.A., DALE-BANNISTER M.A., ADAMS J.B., SMITH M.O., CHRISTENSEN P.R., AND SINGER R.B. (1989) Nature and distribution of surficial deposits in Chryse Planitia and vicinity, Mars. J. Geophys. Res. 94, 1573-1587.
BANIN A. and RISHPON J. (1979) Smectite clays in Mars soil: Evidence for their presence and role in Viking biology experimental results. J. Mol. Evol. 14, 133-152.
BANIN A. and MARGULIES L. (1983) Simulation of Viking biology experiments suggests smectites not palagonites, as martian soil analogs. Nature 305, 523-525.
BANIN A., T. BEN SHLOMO, L. MARGULIES, D.F. BLAKE, R.L. MANCINELLI, and A.U. GEHRING (1993) The nanophase iron mineral(s) in Mars soil. J. Geophys. Res. 98, 20831-20853.
BEER R., NORTON R.H., and MARTONCHIK S.V. (1971) Astronomical infrared spectroscopy with a Connes-type interferometer, 2, Mars, 2500-3500 cmIcarus 15, 1-10.
BELL J.F., III (1992) Charge-Coupled Device Imaging Spectroscopy of Mars. 2. Results and Implications for Martian Ferric Mineralogy. Icarus 100, 575-597.
BELL J.F., III and CRISP D. (1993) Groundbased Imaging Spectroscopy of Mars in the Near-Infrared: Preliminary Results. Icarus 104, 2-19.
BELL J.F., III, MCCORD, T.B. and OWENSBY P.D. (1990a) Observational Evidence of crystalline iron oxides on Mars. J. Geophys. Res. 95, 14447-14461.
BELL J.F., III, MCCORD T.B., and LUCEY P.G. (1990b) Imaging Spectroscopy of Mars (0.4-1.1 um) During the 1988 Opposition. Proc. Lunar Planet. Sci. Conf. XX, 479-486.
BELL J.F., III, POLLACK J.B., GEBALLE T.R., CRUIKSHANK D.P., and FREEDMAN R. (1994a) Spectroscopy of Mars from 2.04 to 2.44 um during the 1993 opposition: Absolute calibration and atmospheric vs. mineralogic origin of narrow absorption features. Icarus 111, 106-123.
BELL J.F., III, MORRIS R.V., and ADAMS J.B. (1994b) Why Mars is red: A process-oriented viewpoint. EOS, Trans. A.G.U. 75, 406.
BELL J.F., III, JAMES P.B., MARTIN L.J., CLANCY R.T., LEE S.W., and CRISP D. (1995) Mars surface mineralogy from Hubble Space Telescope multispectral imaging: 1994 pre-opposition data. Lunar Planet. Sci. Conf. XXVI, 95-96.
BIEMANN K., ORO J., TOULMIN P., III, ORGEL L.E., NIER A.O., ANDERSON D.M., SIMMONDS P.G., FLORY D., DIAZ A.V., RUSHNECK D.R., BILLER J.E., and LAFLEUR A.L. (1977) The search for organic substances and inorganic volatile compounds in the surface of Mars. J. Geophys. Res. 82, 4641-4658.
BINDER A.B. and JONES J.C. (1972) Spectrophotometric studies of the photometric function, composition, and distribution of the surface materials of Mars. J. Geophys. Res. 77, 3005-3020.
BISHOP J.L. AND PIETERS C.M. (1995) Low-temperature and low atmospheric pressure infrared reflectance spectroscopy of Mars soil analog materials. J. Geophys. Res. 100, 5369-5379.
BISHOP J.L., PIETERS C.M., and BURNS R.G. (1993) Reflectance and Mössbauer spectroscopy of ferrihydrite-montmorillonite assemblages as Mars soil analog materials. Geochim. Cosmochim. Acta 57, 4583-4595.
BLANEY D.L. and MCCORD T.B. (1989a) An observational search for carbonates on Mars. J. Geophys. Res. 94, 10159-10166.
BLANEY D.L. AND MCCORD T.B. (1989b) Telescopic spectrometric observations of Mars between 3.5 um and 4.2 um: Evidence for compounds containing H-S. Fourth International Conference on Mars, pp. 71-72, Tucson, AZ, January 10-13, 1989.
BLANEY D.L. and MCCORD T.B. (1995) Indications of sulfate minerals in the Martian soil from Earth-based spectroscopy. J. Geophys. Res., 100, 14433-14442.
BOUSKA V. AND BELL J.F., III (1993) Assumptions about the presence of natural glasses on Mars. J. Geophys. Res. 98, 18719-18726.
BURNS R.G. (1970) Mineralogical Applications of Crystal Field Theory. Cambridge Univ. Press, New York, 224 pp.
BURNS R.G. (1980) Does feroxyhyte occur on the surface of Mars? Nature 285, 647.
BURNS R.G. (1987) Ferric sulfates on Mars. J. Geophys. Res. 92, E570-E574.
BURNS R.G. (1988) Gossans on Mars. Proc. Lunar Planet. Sci. Conf. 18th, 713-721.
BURNS R.G. (1993) Rates and mechanisms of chemical weathering of ferromagnesian silicate minerals on Mars. Geochim. Cosmochim. Acta 57, 4555-4574.
BURNS R.G. and FISHER D.S. (1990) Iron-sulfur mineralogy of Mars: Magmatic evolution and chemical weathering products. J. Geophys. Res. 95, 14415-14421.
BURNS R.G. and FISHER D.S. (1993) Rates of oxidative weathering on the surface of Mars. J. Geophys. Res. 98, 3365-3372 1993.
CALVIN W.M., KING T.V.V., and CLARK R.N. (1994) Hydrous carbonates on Mars?: Evidence from Mariner 6/7 infrared spectrometer and groundbased telescopic spectra. J. Geophys. Res. 99, 14,659-14,676 1994.
CHRISTENSEN P.R., ANDERSON D.L., CHASE S.C., CLARK R.N., KIEFFER H.H., MALIN M.C., PEARL J.C., CARPENTER J., BANDIERA N., BROWN F.G., and SILVERMAN S. (1992), Thermal emission spectrometer experiment: Mars Observer mission. J. Geophys. Res. 97, 7719-7734.
CLANCY R.T., LEE S.W., GLADSTONE G.R., MCMILLAN W.W., and ROUSH T. (1995) A new model for Mars atmospheric dust based upon analysis of ultraviolet through infrared observations from Mariner 9, Viking, and Phobos. J. Geophys. Res. 100, 5251-5264.
CLARK B.C., BAIRD A.K., WELDON R.J., TSUSAKI D.M., SCHNABEL L., and CANDELARIA M.P. (1982) Chemical composition of Martian fines. J. Geophys. Res. 87, 10059-10067.
CLARK R.N., SWAYZE G.A., SINGER R.B. and POLLACK J.B. (1990) High-resolution reflectance spectra of Mars in the 2.3 um region: Evidence for the mineral scapolite. J. Geophys. Res. 95, 14463-14480.
DE VAUCOULEURS G. (1954) Physics of the Planet Mars. Faber and Faber, London, 365 pp.
DREIBUS G. and WäNKE H., Mars: a volatile rich planet. Meteoritics 20, 367-382 1985.
ENCRENAZ T. and LELLOUCH E. (1990) On the atmospheric origin of weak absorption features in the infrared spectrum of Mars. J. Geophys. Res. 95, 14589-14593.
FANALE F.P., SALVAIL J.R., BANERDT W.B., and SAUNDERS R.S. (1982) Mars: The regolith-atmosphere-cap system and climate change. Icarus 50, 381.
FANALE F.P., POSTAWKO S.E., POLLACK J.B., CARR M.H., and PEPIN R.O. (1992) Mars: Epochal climate change and volatile history. In Mars (ed. H. H. Kieffer, B.M. Jakosky, C.W. Snyder, and M.S. Matthews), pp. 1135-1179, Univ. Arizona Press, Tucson.
FARMER V.C. (1974) The anhydrous oxide minerals. In The Infrared Spectra of Minerals (ed. V.C. Farmer), pp. 183-204, Mineral. Soc. Monograph 4, Mineral. Soc., London.
FARMER C.B., DAVIES D.W., HOLLAND A.L., LA PORTE D.D., and DOMS P.E. (1977) Mars: Water vapor observations from the Viking orbiters. J. Geophys. Res. 82, 4225-4248.
FISCHER E.M. and PIETERS C.M. (1993) The continuum slope of Mars: Bidirectional reflectance investigations and applications to Olympus Mons. Icarus 102, 185-202.
GAFFEY S.J., MCFADDEN L.A., and NASH D.B. (1993) Ultraviolet, visible, and near-infrared reflectance spectroscopy: Laboratory spectra of geologic materials. In Remote Geochemical Analysis: Elemental and Mineralogical Composition (ed. C. Pieters and P. Englert), pp. 43-71. Cambridge Univ. Press.
GEISSLER P.E., SINGER R.B., KOMATSU G., MURCHIE S., and MUSTARD J. (1993) An unusual spectral unit in West Candor Chasma: Evidence for aqueous or hydrothermal alteration in the Martian canyons. Icarus 106, 380-391.
GOODING J.L. (1978) Chemical weathering on Mars. Icarus 33, 483-513.
GOODING J.L. (1992) Soil mineralogy and chemistry on Mars: Possible clues from salts and clays in SNC meteorites. Icarus 99, 28-41.
GOODING J.L. and KEIL K. (1978) Alteration of glass as a possible source of clay minerals on Mars. Geophys. Res. Lett. 5, 727-730.
GOODING J.L. and MUENOW D.W. (1986) Martian volatiles in shergottite EETA 79001: New evidence from oxidized sulfur and sulfur-rich aluminosilicates. Geochim. Cosmochim Acta 50, 1049-1059.
GOODING J.L., ARVIDSON R.E., AND ZOLOTOV M.Y. (1992) Physical and Chemical Weathering. In Mars (ed. H. H. Kieffer, B.M. Jakosky, C.W. Snyder, and M.S. Matthews), pp. 626-651, Univ. Arizona Press, Tucson.
GUINNESS E.A., ARVIDSON R.E., DALE-BANNISTER M.A., SINGER R.B. AND BRUCKENTHAL E.A. (1987) On the spectral reflectance properties of materials exposed at the Viking landing sites. J. Geophys. Res., 92, E575-E587.
HARDORP J. (1978) The Sun among the stars. I. A search for solar spectral analogs. Astron. Astrophys. 63, 383-390.
HARGRAVES R.B., COLLINSON D.W., ARVIDSON R.E., and SPITZER C.R. (1977) The Viking magnetic properties experiment: Primary mission results. J. Geophys. Res. 82, 4547-4558.
HOUCK J.R., POLLACK J.B., SAGAN C., SCHAACK D., and DECKER J. (1973) High altitude infrared spectroscopic evidence for bound water on Mars. Icarus 18, 470-480.
HUBBARD J.S. (1979) Laboratory simulation of the Pyrolitic Release experiment. J. Molec. Evol. 14, 211-222.
HUGUENIN R.L. (1987) The silicate component of martian dust. Icarus 70, 162-168.
HUNT G.R. and SALISBURY J.W. (1971) Visible and near-infrared spectra of minerals and rocks: II. Carbonates. Mod. Geol. 2, 23-30.
HUNT G.R. and SALISBURY J.W. and LENHOFF C.J. (1971) Visible and near-infrared spectra of minerals and rocks: III. Oxides and Hydroxides. Mod. Geol. 2, 195-205.
HUNT G.R., LOGAN L.M., and SALISBURY J.W. (1973) Mars: Components of infrared spectra and the composition of the dust cloud. Icarus 18, 459-469.
JAKOSKY B.M. and FARMER C.B. (1982) The seasonal and global behavior of water vapor in the Mars atmosphere: Complete global results of the Viking atmospheric water detector experiment. J. Geophys. Res. 87, 2999-3019.
JAMES P.B., CLANCY R.T., LEE S., MARTIN L.J., SINGER R.B., SMITH E., KAHN R., and ZUREK R. (1994) Monitoring Mars with the Hubble Space Telescope: 1990-1991 observations. Icarus 109, 79-101.
KASTING J.F. (1991) CO2 condensation and the climate of early Mars. Icarus 94, 1-13.
KUHN W.R. and ATREYA S.K. (1979) Ammonia photolysis and the greenhouse effect in the primordial atmosphere of the Earth. Icarus 37, 207-213.
LAUL J. C., SMITH M.R., WäNKE H., JAGOUTZ E., DREIBUS G., PALME H., SPETTEL B., BURGHELE A., LIPSCHITZ M.E., AND VERKOUTERAN R.M. (1986) Chemical systematics of the Shergotty meteorite and the composition of its parent body (Mars). Geochim. Cosmochim. Act, 50, 909-926.
MCCARTHY T.S., ERLANK A.J., WILLIS J.P., AND AHRENS L.H. (1974) New chemical analyses of six achondrites and one chondrite. Meteoritics 9, 215-222.
MCCORD T.B. and ADAMS J.B. (1969) Spectral reflectivity of Mars. Science 163, 1058-1060.
MCCORD T.B. and WESTPHAL J.A. (1971) Mars: Narrowband photometry, from 0.3 to 2.5 microns, of surface regions during the 1969 apparition. Astrophys. J. 168, 141-153.
MCCORD T.B., HUGUENIN R.L., MINK D., AND PIETERS C. (1977) Spectral reflectance of Martian areas during the 1973 opposition: Photoelectric filter photometry, 0.33-1.10 um. Icarus 31, 25-39.
MCCORD T.B., CLARK R.N., and HUGUENIN R.L. (1978) Mars: Near-infrared spectral reflectance and compositional implications. J. Geophys. Res. 83, 5433-5441.
MCCORD T.B., CLARK R.N. and SINGER R.B. (1982a) Mars: Near-infrared spectral reflectance of surface regions and compositional implications. J. Geophys. Res. 87, 3021-3032.
MCCORD T.B., SINGER R.B., HAWKE B.R., ADAMS J.B., EVANS D.L., HEAD J.W., MOUGINIS-MARK P.J., PIETERS C.M., HUGUENIN R.L., and ZISK S.H. (1982b) Mars: Definition and characterization of global surface units with emphasis on composition. J. Geophys. Res. 87, 10129-10148.
MCKAY C.P. and NEDELL S.S. (1988) Are there carbonate deposits in the Valles Marineris. Mars? Icarus 73, 142-148.
MCSWEEN H.Y., Jr. (1994) What we have learned about Mars from SNC meteorites. Meteoritics 29, 757-779.
MOERSCH J., NICHOLSON P., SQUYRES S., VAN CLEVE J., LEE P., HAYWARD T., HOUCK J., and MILES J. (1993), Thermal infrared observations of Mars during the 1993 opposition. Bull. Amer. Astron. Soc. 25, 1033.
MORRIS R.V., LAUER H.V., Jr., LAWSON C.A., GIBSON E.K., Jr., NACE G.A., and STEWART C. (1985) Spectral and other physicochemical properties of submicron powders of hematite ([[alpha]]-Fe2O3), maghemite ([[gamma]]-Fe2O3), magnetite (Fe3O4), goethite ([[alpha]]-FeOOH), and lepidocrocite ([[gamma]]-FeOOH). J. Geophys. Res. 90, 3126-3144.
MORRIS R.V., GOLDEN D.C., BELL J.F., III, and LAUER H.V., Jr. (1995) Hematite, pyroxene, and phyllosilicates on Mars: Implications from oxidized impact melt rocks from Manicouagan Crater, Quebec, Canada. J. Geophys. Res. 100, 5319-5329.
MORRIS R.V., MING D.W., GOLDEN D.C., AND BELL J.F., III (1996) An occurrence of jarositic tephra on Mauna Kea, Hawaii: Implications for the ferric mineralogy of the Martian surface. This volume.
MURCHIE S., MUSTARD J., BISHOP J., HEAD J., PIETERS C., and ERARD S. (1993) Spatial variations in the spectral properties of bright regions on Mars. Icarus 105, 454-468.
MURCHIE S., MERéNYI E., SINGER R., AND KIRKLAND L. (1996) Visible-NIR spectroscopic evidence for the composition of low-albedo altered soils on Mars, Lunar and Planetary Science XXVI, in press.
MUSTARD J.F. and BELL J.F., III (1994) New composite reflectance spectra of Mars from 0.4 to 3.14 um. Geophys. Res. Lett. 21, 353-356.
MUSTARD J.F. and SUNSHINE J.M. (1995) Seeing through the dust: Martian crustal heterogeneity and links to the SNC meteorites. Science 267, 1623-1626.
MUSTARD J.F., ERARD S., BIBRING J.-P., HEAD J.W., HURTREZ S., LANGEVIN Y., PIETERS C.M., and SOTIN C.J. (1993) The surface of Syrtis Major: Composition of the volcanic substrate and mixing with altered dust and soil. J. Geophys. Res. 98, 3387-3400.
O'CONNOR J.T. (1968) Mineral stability at the Martian surface. J. Geophys. Res. 73, 5301-5311.
PIMENTEL G.C., FORNEY P.B., and HERR K.C. (1974) Evidence about hydrate and solid water in the Martian surface from the 1969 Mariner infrared spectrometer. J. Geophys. Res. 79, 1623-1634.
POLLACK J.B., COLBURN D., KAHN R., HUNTER J., VAN CAMP W., and BALDWIN B. (1977) Properties of aerosols in the Martian atmosphere, as inferred from Viking Lander data. J. Geophys. Res. 82, 4479-4496.
POLLACK J.B., KASTING J.F., RICHARDSON S.M., and POLIAKOFF K. 1987. The case for a wet, warm climate on Mars. Icarus 71, 203-224.
POLLACK J.B., ROUSH T.L., WITTEBORN F., BREGMAN J., WOODEN D., STOKER C., TOON O.B., RANK D., DALTON B., and FREEDMAN R. (1990) Thermal emission spectra of Mars (5.4- 10.5 um): Evidence for sulfates, carbonates, and hydrates. J. Geophys. Res. 95, 14,595- 14,627.
POSTAWKO S.E. and KUHN W.R. (1986) Effect of the greenhouse gases (CO2, H2O, SO2) on Martian paleoclimate. J. Geophys. Res. 91, D431-D438.
ROUSH T.L. and BELL J.F., III (1995) Thermal emission measurements (5-25 um, 2000-400 cm-1) of Hawaiian palagonitic soils and their implications for Mars, J. Geophys. Res. 100, 5309-5318.
ROUSH T.L., BLANEY D., MCCORD T.B., and SINGER R.B. (1986) Carbonates on Mars: Searching the Mariner 6 and 7 IRS measurements. Lunar Planet. Sci. XVII, 732-733.
ROUSH T.L., BLANEY D.L., and SINGER R.B. (1993) The surface composition of Mars as inferred from spectroscopic observations. In Remote Geochemical Analysis: Elemental and Mineralogical Composition, (ed. C. Pieters and P. Englert), pp. 367-393. Cambridge Univ. Press.
ROWLAND C.M., ROUSH T.L., SLOAN G.C., and BELL J.F., III (1995) Thermal infrared (7-14 um) spectral imaging of Mars. Lunar Planet. Sci. Conf. XXVI, 1195-1196.
SALISBURY J.W., WALTER L.S., VERGO N., and D'ARIA D.M. (1991) Infrared (2.1-25 um) Spectra of Minerals. Johns Hopkins Univ. Press, Baltimore, 267 pp.
SCHAEFER M.W. (1990) Geochemical evolution of the northern plains of Mars: Early hydrosphere, carbonate development, and present morphology. J. Geophys. Res. 95, 14,291-14,300.
SCHAEFER M.W. (1993) Aqueous geochemistry on early Mars. Geochim. Cosmochim. Acta 57, 4619-4625.
SHERMAN D.M. and WAITE T.D. (1985) Electronic spectra of Fe3+ oxides and oxide hydroxides in the near-IR to near-UV. Amer. Mineral. 70, 1262-1269.
SHERMAN D.M., BURNS R.G., and BURNS V.M. (1982) Spectral characteristics of the iron oxides with application to the martian bright region mineralogy. J. Geophys. Res. 87, 10169-10180.
SINGER R.B. (1982) Spectral evidence for the mineralogy of high-albedo soils and dust on Mars. J. Geophys. Res. 87, 10159-10168.
SINGER R.B. and ROUSH T.L. (1983) Spectral reflectance properties of particulate weathered coatings on rocks: Laboratory modeling and applicability to Mars. Lunar Planet. Sci. XIV, 708-709.
SINGER R.B., MCCORD T.B., CLARK R.N., ADAMS J.B., and HUGUENIN R.L. (1979) Mars surface composition from reflectance spectroscopy: A summary. J. Geophys. Res. 84, 8415-8426.
SINGER R.B., MILLER J.S., and WELLS W.K. (1990) Observed variations in martian crustal composition (abstract). Bull. Amer. Astron. Soc. 22, 1061.
SINTON W.M. (1967) On the composition of martian surface materials. Icarus 6, 222-228.
SODERBLOM L.A. (1992) The composition and mineralogy of the martian surface from spectroscopic observations: 0.3-50 um. In Mars (ed. H. H. Kieffer, B.M. Jakosky, C.W. Snyder, and M.S. Matthews), pp. 557-593. Tucson: Univ. Arizona Press.
SQUYRES S.W. and KASTING J.F. (1994) Early Mars: How warm and how wet? Science, 265, 744-749.
SWAYZE G.A., and CLARK R.N. (1990) Infrared spectra and crystal chemistry of scapolites: Implications for Martian mineralogy. J. Geophys. Res. 95, 14481-14495.
TOON O.B., POLLACK J.B., and SAGAN C. (1977) Physical properties of the particles composing the Martian dust storm of 1971-1972. Icarus 30, 663-696.
TOULMIN P., III, BAIRD A.K., CLARK B.C., KEIL K., ROSE H.J., Jr., CHRISTIAN R.P., EVANS P.H., and KELLIHER W.C. (1977) Geochemical and Mineralogical Interpretation of the Viking inorganic chemical results. J. Geophys. Res. 82, 4625-4634.
TREIMAN A.H., BARRETT R.A., and GOODING J.L. (1993) Preterrestrial aqueous alteration of the Lafayette (SNC) meteorite. Meteoritics 28, 86-97.
FIG. 1: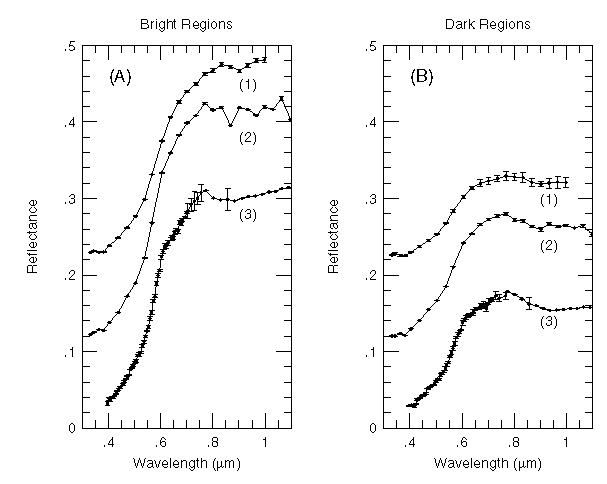
FIG. 2A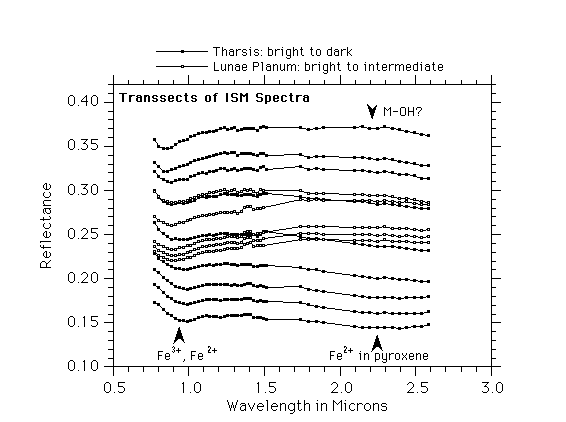
FIG. 2B
FIG. 3A:

 FIG. 3B Part 1: and
FIG. 3B Part 2:
Modified Gaussian Model (MGM) analysis of
ISM spectra of the dark regions Eos in Valles Marineris and Nili Patera in
Syrtis Major (top frame) from MUSTARD and SUNSHINE (1995). The Eos spectrum
was modeled (middle frame) as a combination of low-calcium pyroxene (LCP) and
high-calcium pyroxene (HCP), and the Nili spectrum was modeled (bottom frame)
as a combination of LCP, HCP, and a ferric-bearing component. The relative
strengths of the modeled pyroxene absorptions indicate a ~20% increase in HCP
in Nili relative to Eos.
FIG. 3B Part 1: and
FIG. 3B Part 2:
Modified Gaussian Model (MGM) analysis of
ISM spectra of the dark regions Eos in Valles Marineris and Nili Patera in
Syrtis Major (top frame) from MUSTARD and SUNSHINE (1995). The Eos spectrum
was modeled (middle frame) as a combination of low-calcium pyroxene (LCP) and
high-calcium pyroxene (HCP), and the Nili spectrum was modeled (bottom frame)
as a combination of LCP, HCP, and a ferric-bearing component. The relative
strengths of the modeled pyroxene absorptions indicate a ~20% increase in HCP
in Nili relative to Eos.
FIG. 4A:
FIG. 4B: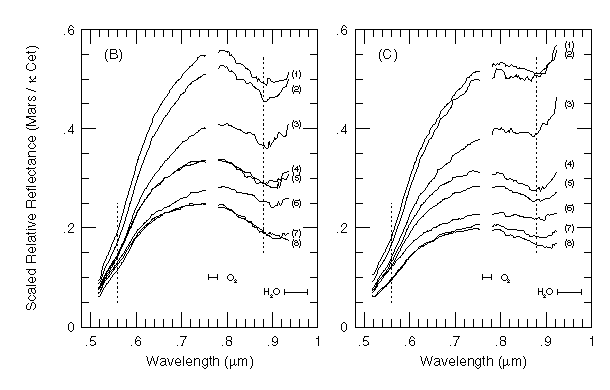
FIG. 5A:
FIG 5B:
FIG. 6:
FIG. 7:
Last Modified by Jim Bell on 27 February 1996.